Starch Can Be Broken Down Into the Disaccharide Known as _____.
Most people are familiar with carbohydrates, one type of macromolecule, particularly when it comes to what nosotros consume. To lose weight, some individuals adhere to "low-carb" diets. Athletes, in contrast, oft "carb-load" before important competitions to ensure that they have enough free energy to compete at a high level. Carbohydrates are, in fact, an essential part of our nutrition; grains, fruits, and vegetables are all natural sources of carbohydrates. Carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many staple foods. Carbohydrates also have other important functions in humans, animals, and plants.
Molecular Structures
Carbohydrates tin be represented past the stoichiometric formula (CHiiO)n, where northward is the number of carbons in the molecule. In other words, the ratio of carbon to hydrogen to oxygen is ane:2:1 in sugar molecules. This formula also explains the origin of the term "carbohydrate": the components are carbon ("carbo") and the components of water (hence, "hydrate"). Carbohydrates are classified into 3 subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono– = "ane"; sacchar– = "sweet") are simple sugars, the most common of which is glucose. In monosaccharides, the number of carbons ordinarily ranges from three to 7. Most monosaccharide names finish with the suffix –ose. If the saccharide has an aldehyde group (the functional grouping with the structure R-CHO), it is known as an aldose, and if it has a ketone group (the functional group with the structure RC(=O)R'), it is known equally a ketose. Depending on the number of carbons in the sugar, they likewise may be known equally trioses (iii carbons), pentoses (five carbons), and or hexoses (half-dozen carbons). Run across Figure ane for an illustration of the monosaccharides.

Effigy 1. Monosaccharides are classified based on the position of their carbonyl group and the number of carbons in the backbone. Aldoses have a carbonyl group (indicated in green) at the terminate of the carbon chain, and ketoses have a carbonyl group in the middle of the carbon chain. Trioses, pentoses, and hexoses have iii, five, and six carbon backbones, respectively.
The chemical formula for glucose is CsixH12Ovi. In humans, glucose is an important source of free energy. During cellular respiration, energy is released from glucose, and that energy is used to help make adenosine triphosphate (ATP). Plants synthesize glucose using carbon dioxide and water, and glucose in turn is used for energy requirements for the plant. Backlog glucose is often stored as starch that is catabolized (the breakdown of larger molecules by cells) past humans and other animals that feed on plants.
Galactose (part of lactose, or milk sugar) and fructose (part of sucrose, or fruit sugar) are other common monosaccharides. Although glucose, galactose, and fructose all accept the aforementioned chemical formula (CsixH12Ovi), they differ structurally and chemically (and are known as isomers) because of the dissimilar arrangement of functional groups around the asymmetric carbon; all of these monosaccharides take more than one disproportionate carbon (Figure 2).
Practise
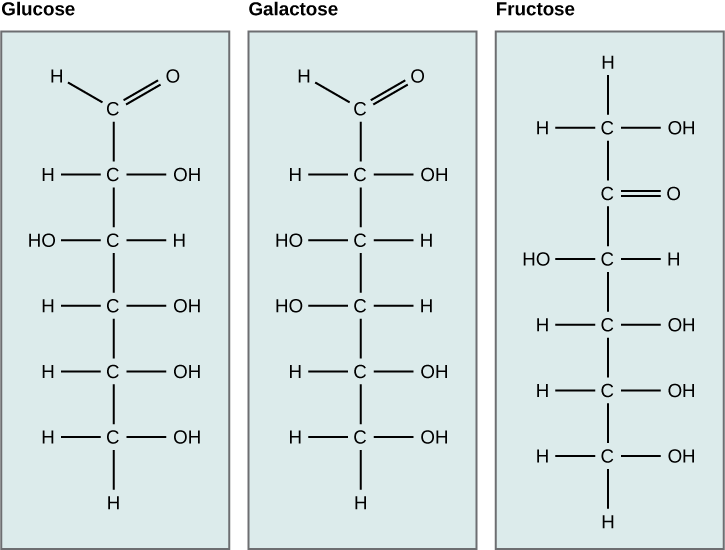
Figure 2. Glucose, galactose, and fructose are all hexoses. They are structural isomers, meaning they have the aforementioned chemical formula (C6H12O6) but a dissimilar arrangement of atoms.
What kind of sugars are these, aldose or ketose?
Click to Show Answer
Glucose and galactose are aldoses. Fructose is a ketose.
Monosaccharides can exist as a linear chain or as band-shaped molecules; in aqueous solutions they are usually institute in band forms (Effigy 3). Glucose in a ring form can have two different arrangements of the hydroxyl grouping (-OH) around the anomeric carbon (carbon 1 that becomes asymmetric in the process of band formation). If the hydroxyl grouping is below carbon number 1 in the carbohydrate, it is said to be in the blastoff (α) position, and if information technology is to a higher place the plane, it is said to be in the beta (β) position.

Figure three. V and six carbon monosaccharides exist in equilibrium between linear and band forms. When the ring forms, the side chain it closes on is locked into an α or β position. Fructose and ribose likewise form rings, although they form five-membered rings equally opposed to the half dozen-membered ring of glucose.
Disaccharides
Disaccharides (di– = "two") form when two monosaccharides undergo a dehydration reaction (also known as dehydration synthesis). During this procedure, the hydroxyl grouping of 1 monosaccharide combines with the hydrogen of another monosaccharide, releasing a molecule of h2o and forming a covalent bond. A covalent bail formed between a carbohydrate molecule and another molecule (in this case, between two monosaccharides) is known as a glycosidic bond (Effigy 4). Glycosidic bonds (likewise chosen glycosidic linkages) tin can be of the alpha or the beta type.

Figure iv. Sucrose is formed when a monomer of glucose and a monomer of fructose are joined in a dehydration reaction to form a glycosidic bail. In the procedure, a h2o molecule is lost. By convention, the carbon atoms in a monosaccharide are numbered from the final carbon closest to the carbonyl group. In sucrose, a glycosidic linkage is formed between carbon 1 in glucose and carbon two in fructose.
Common disaccharides include lactose, maltose, and sucrose (Figure 5). Lactose is a disaccharide consisting of the monomers glucose and galactose. It is found naturally in milk. Maltose, or malt saccharide, is a disaccharide formed by a dehydration reaction between 2 glucose molecules. The most common disaccharide is sucrose, or table carbohydrate, which is composed of the monomers glucose and fructose.

Figure v. Mutual disaccharides include maltose (grain carbohydrate), lactose (milk sugar), and sucrose (table carbohydrate).
Polysaccharides
A long chain of monosaccharides linked past glycosidic bonds is known as apolysaccharide (poly– = "many"). The concatenation may be branched or unbranched, and it may contain different types of monosaccharides. The molecular weight may be 100,000 daltons or more depending on the number of monomers joined. Starch, glycogen, cellulose, and chitin are principal examples of polysaccharides.
Starch is the stored course of sugars in plants and is made upwards of a mixture of amylose and amylopectin (both polymers of glucose). Plants are able to synthesize glucose, and the excess glucose, beyond the constitute's immediate free energy needs, is stored as starch in different plant parts, including roots and seeds. The starch in the seeds provides food for the embryo as it germinates and can also human action every bit a source of food for humans and animals. The starch that is consumed by humans is cleaved down by enzymes, such equally salivary amylases, into smaller molecules, such as maltose and glucose. The cells can then absorb the glucose.
Starch is made upwardly of glucose monomers that are joined byα one-4 or α 1-vi glycosidic bonds. The numbers 1-iv and 1-6 refer to the carbon number of the two residues that have joined to form the bail. Equally illustrated in Figure six, amylose is starch formed past unbranched chains of glucose monomers (only α ane-4 linkages), whereas amylopectin is a branched polysaccharide (α one-6 linkages at the co-operative points).

Effigy 6. Amylose and amylopectin are two different forms of starch. Amylose is composed of unbranched chains of glucose monomers connected past α ane,4 glycosidic linkages. Amylopectin is composed of branched chains of glucose monomers connected by α 1,4 and α ane,six glycosidic linkages. Because of the manner the subunits are joined, the glucose chains have a helical construction. Glycogen (non shown) is similar in structure to amylopectin but more than highly branched.
Glycogen is the storage class of glucose in humans and other vertebrates and is made upward of monomers of glucose. Glycogen is the animal equivalent of starch and is a highly branched molecule ordinarily stored in liver and muscle cells. Whenever blood glucose levels decrease, glycogen is broken down to release glucose in a process known as glycogenolysis.
Cellulose is the almost abundant natural biopolymer. The cell wall of plants is generally made of cellulose; this provides structural back up to the cell. Wood and paper are by and large cellulosic in nature. Cellulose is fabricated up of glucose monomers that are linked byβ ane-iv glycosidic bonds (Figure vii).

Figure seven. In cellulose, glucose monomers are linked in unbranched chains by β ane-4 glycosidic linkages. Because of the way the glucose subunits are joined, every glucose monomer is flipped relative to the next 1 resulting in a linear, fibrous construction.
Every bit shown in Figure seven, every other glucose monomer in cellulose is flipped over, and the monomers are packed tightly equally extended long chains. This gives cellulose its rigidity and loftier tensile strength—which is so important to plant cells. While theβ i-four linkage cannot be broken downwards by man digestive enzymes, herbivores such as cows, koalas, buffalos, and horses are able, with the aid of the specialized flora in their stomach, to assimilate plant material that is rich in cellulose and use it as a food source. In these animals, certain species of bacteria and protists reside in the rumen (part of the digestive organisation of herbivores) and secrete the enzyme cellulase. The appendix of grazing animals besides contains bacteria that digest cellulose, giving information technology an important role in the digestive systems of ruminants. Cellulases tin break down cellulose into glucose monomers that tin can be used equally an energy source by the animal. Termites are also able to pause downwards cellulose considering of the presence of other organisms in their bodies that secrete cellulases.

Figure eight. Insects have a difficult outer exoskeleton made of chitin, a type of polysaccharide. (credit: Louise Docker)
Carbohydrates serve various functions in different animals. Arthropods (insects, crustaceans, and others) accept an outer skeleton, called the exoskeleton, which protects their internal body parts (as seen in the bee in Figure 8). This exoskeleton is made of the biological macromolecule chitin, which is a polysaccharide-containing nitrogen. Information technology is fabricated of repeating units of Due north-acetyl-β-d-glucosamine, a modified sugar. Chitin is also a major component of fungal cell walls; fungi are neither animals nor plants and form a kingdom of their ain in the domain Eukarya.
Section Summary
Carbohydrates are a group of macromolecules that are a vital energy source for the cell and provide structural support to plant cells, fungi, and all of the arthropods that include lobsters, crabs, shrimp, insects, and spiders. Carbohydrates are classified as monosaccharides, disaccharides, and polysaccharides depending on the number of monomers in the molecule. Monosaccharides are linked by glycosidic bonds that are formed as a result of dehydration reactions, forming disaccharides and polysaccharides with the emptying of a h2o molecule for each bond formed. Glucose, galactose, and fructose are common monosaccharides, whereas common disaccharides include lactose, maltose, and sucrose. Starch and glycogen, examples of polysaccharides, are the storage forms of glucose in plants and animals, respectively. The long polysaccharide chains may be branched or unbranched. Cellulose is an example of an unbranched polysaccharide, whereas amylopectin, a constituent of starch, is a highly branched molecule. Storage of glucose, in the form of polymers like starch of glycogen, makes it slightly less attainable for metabolism; all the same, this prevents it from leaking out of the cell or creating a high osmotic pressure that could cause excessive water uptake by the cell.
Bank check Your Understanding
Reply the question(s) below to see how well you understand the topics covered in the previous section. This curt quiz doesnot count toward your grade in the class, and you can retake it an unlimited number of times.
Apply this quiz to check your understanding and decide whether to (1) written report the previous section further or (2) motility on to the side by side department.
vanleuvenwaallovar.blogspot.com
Source: https://courses.lumenlearning.com/ivytech-bio1-1/chapter/reading-types-of-carbohydrates/
Post a Comment for "Starch Can Be Broken Down Into the Disaccharide Known as _____."